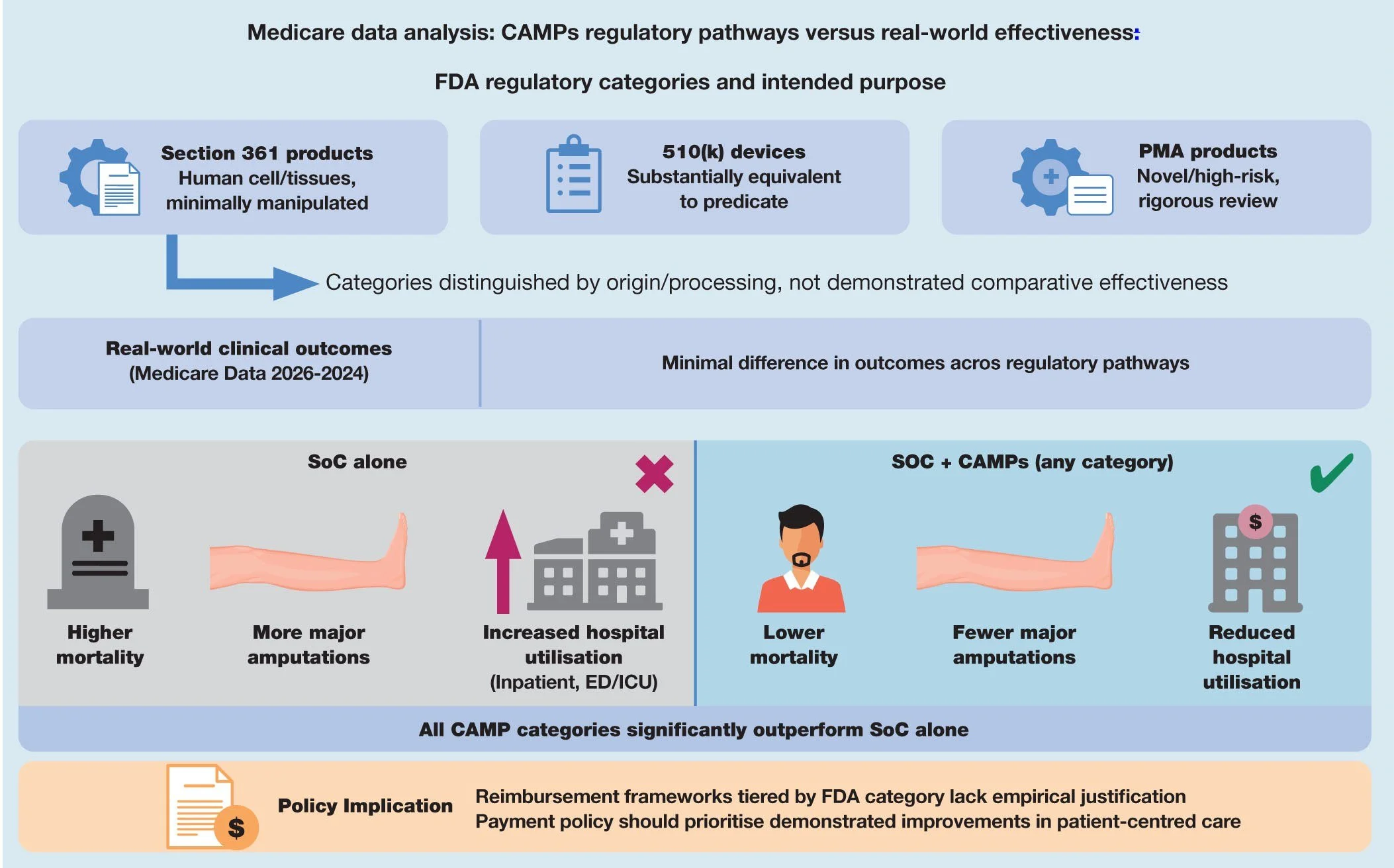
JWC: Rethinking regulatory tiers: Medicare real-world evidence shows CAMP outcomes are independent of FDA regulatory classification
Cellular, acellular, and matrix-like products (CAMPs) are widely used for treating hard-to-heal lower-extremity diabetic ulcers (LEDUs) and venous leg ulcers (VLUs); yet, limited evidence exists to demonstrate that clinical outcomes differ across the US Food and Drug Administration's (FDA) regulatory categories: section 361 products; 510(k) devices; and premarket approval products. Despite substantial differences in evidentiary requirements, the FDA's regulatory categories largely distinguish CAMPs by their origin, degree of manipulation and intended purpose, rather than by demonstrated comparative effectiveness. The Centers for Medicare and Medicaid Services' (CMS) commissioned technology assessments and systematic reviews similarly report insufficient evidence that any specific CAMP category consistently outperforms another. Prospective randomised ‘controlled’ trials rarely include the complex, multimorbid populations seen in routine practice and ‘real-world’ data, whether collected prospectively or retrospectively. Yet routine practice and real-world data are essential for understanding how these technologies perform in actual Medicare beneficiaries. This study evaluated whether regulatory classification predicts real-world clinical effectiveness and how CAMP-treated episodes compare with standard of care (SoC) alone.
Authors: William H Tettelbach, MD, FACP, FIDSA, FUHM, MAPWCA, CHWS tarpon@xmission.com, David G Armstrong, DPM, MD, PhD, Travis Tucker, MA, MBA, Thomas A Davenport, MD, Lynnette A Morrison, MD, David B Alper, DPM, FFPM RCPS, Jonathan Johnson, MD, MBA, CWSP, FAPWCA, Lee C Rogers, DPM, FFPM RCPS, Jeffery A Niezgoda, MD, FACHM, MAPWCA, CHWS, Jennie Feight, MS, CPC, CPMA, CPC-I, Kevin Nolan, MD, MPH, FACS, Daniel Kapp, MD, Naz Wahab, MD, FAAFP, MAPWCA, and Martha R Kelso, RN, CHWS, DAPWCA, HBOT